Beautiful Work Tips About How To Build A Chlorine Atom

The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and.
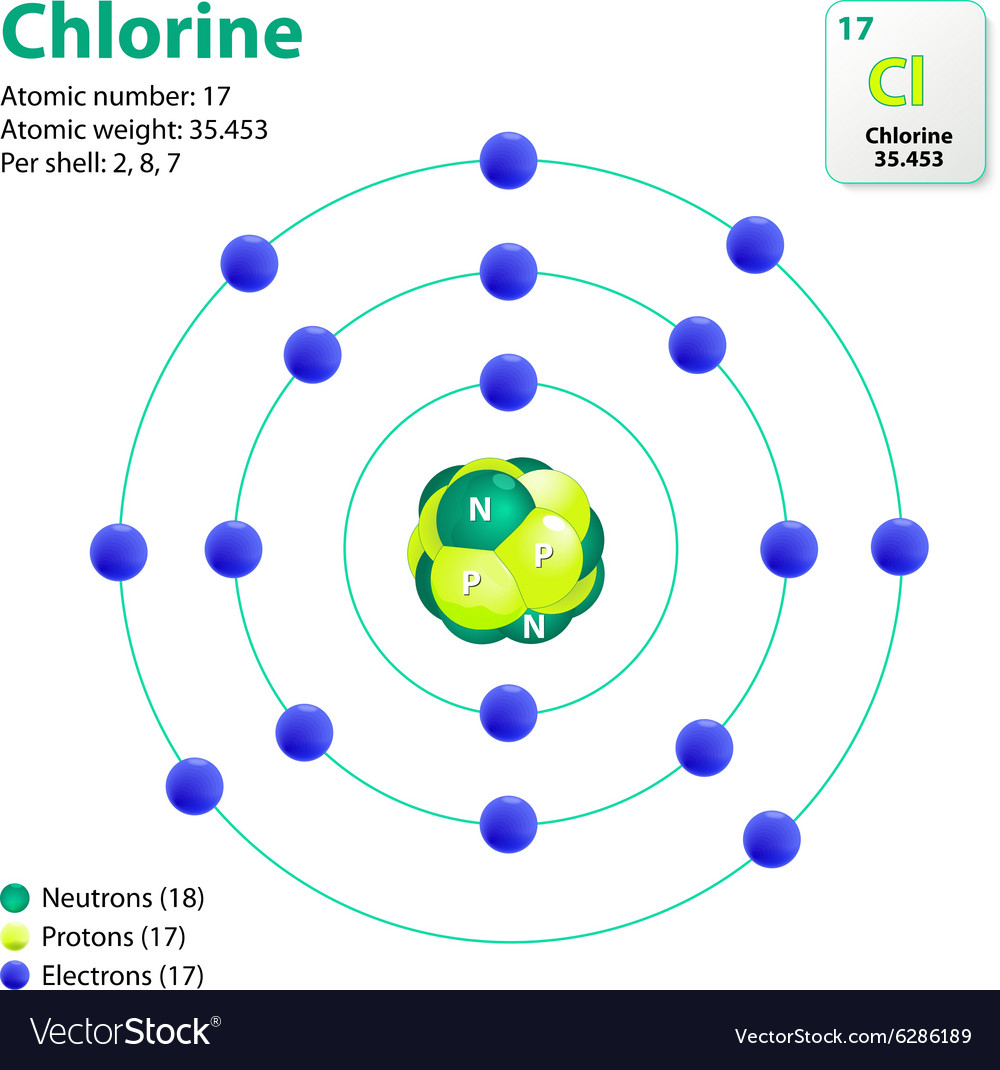
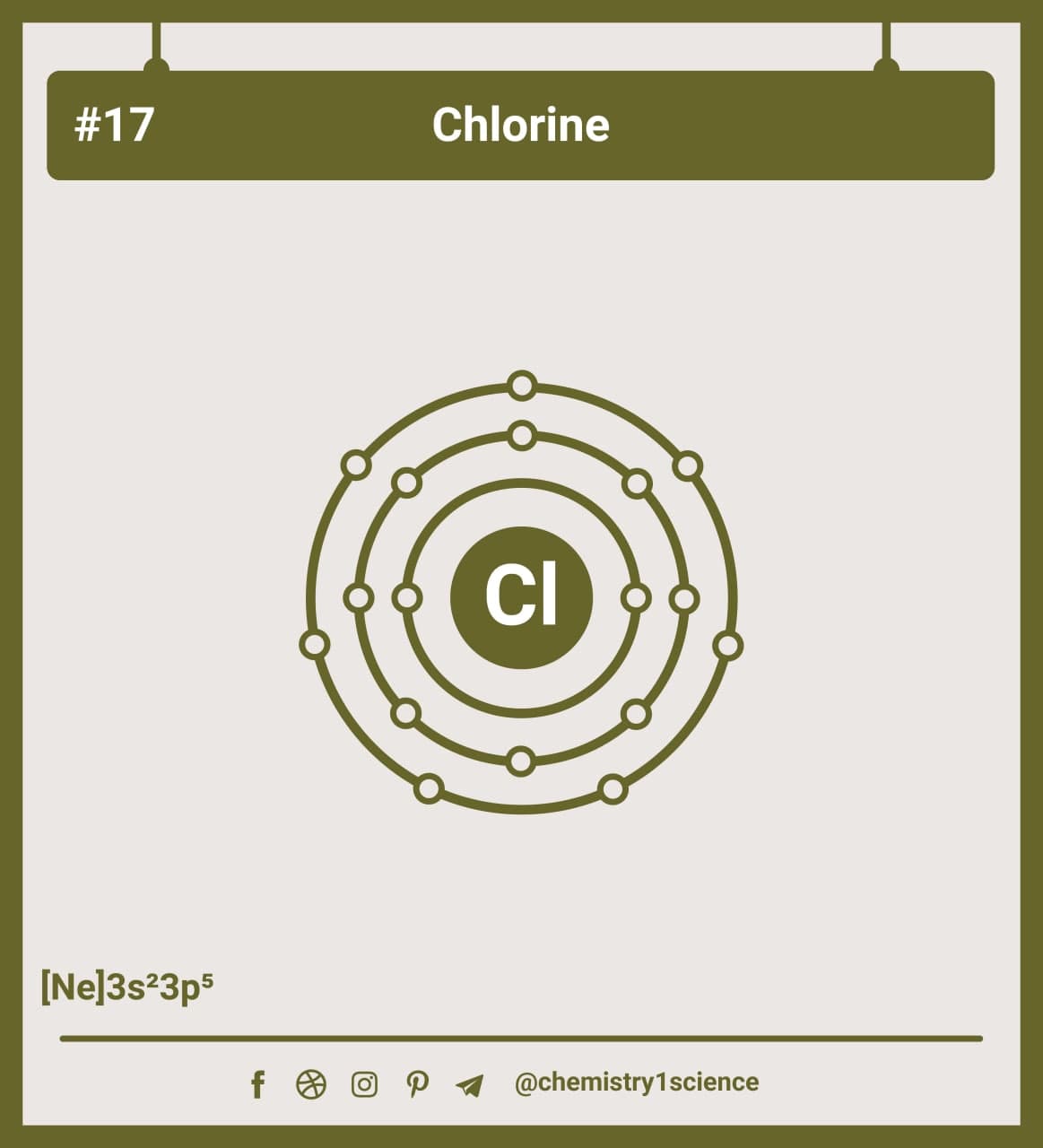
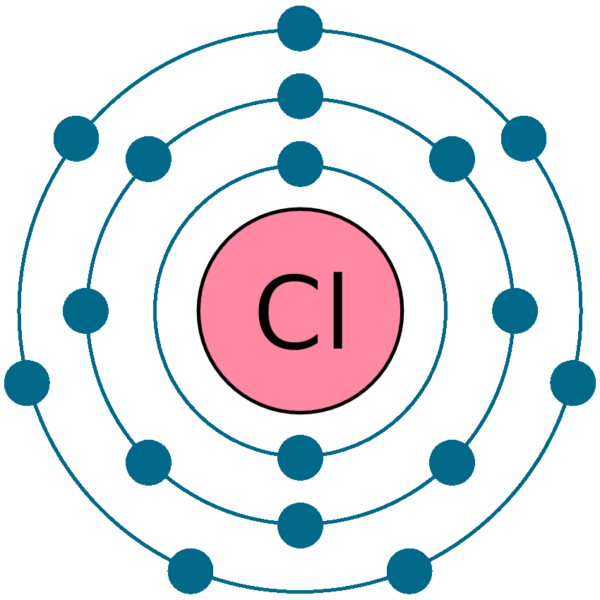
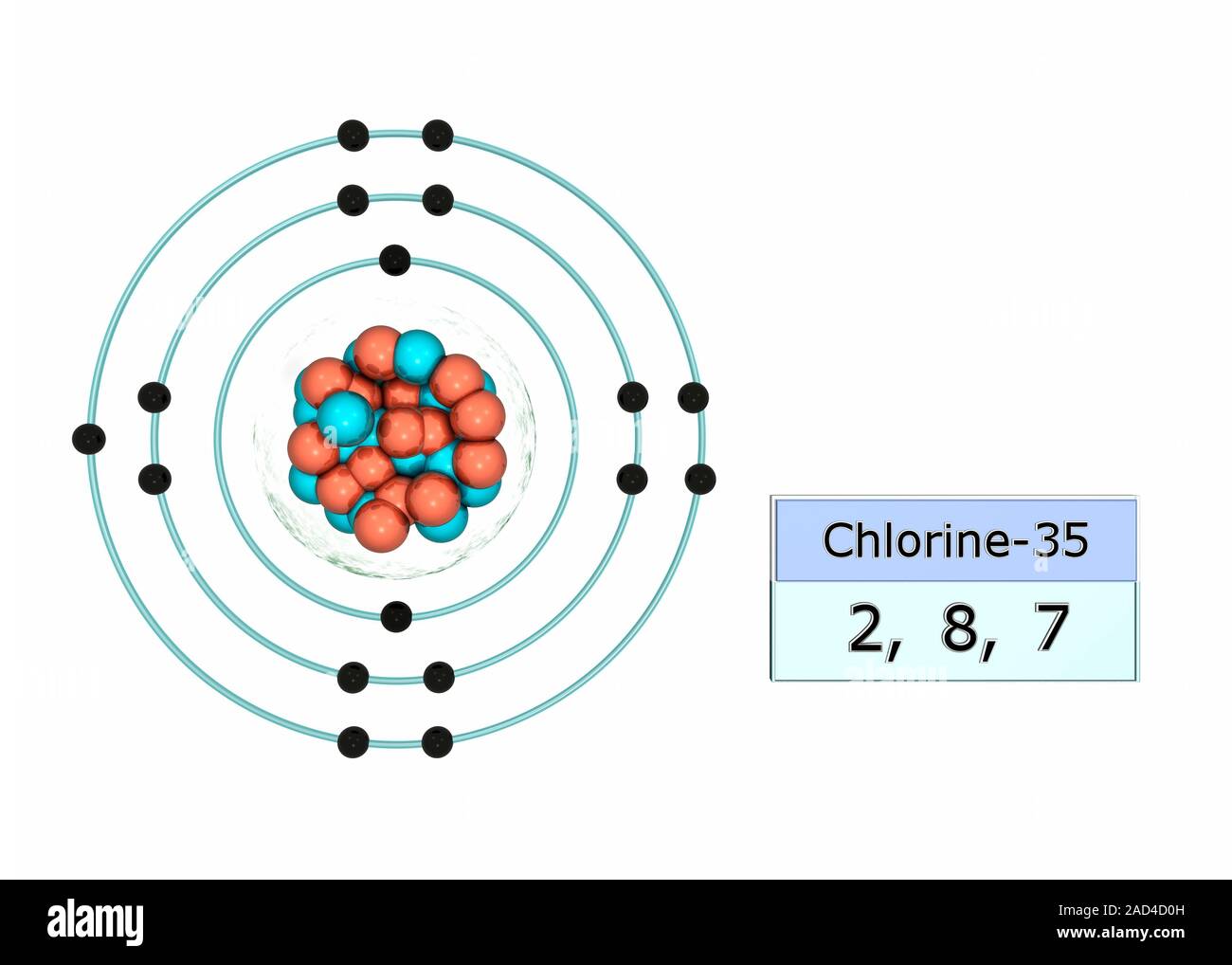
How to build a chlorine atom. Chlorine atoms have two electrons in the inner ring, then eight electrons in the. Color all of the other dots in that chlorine atom green.
Make a simple germy liquid removal using chlorine. In the first of these, the photochemical substitution. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions.
Build a chlorine atom. Build a chlorine atom by placing the right numers of electrons, protons and neutrons in their respective places. The liquid piping is as shown.
Notice that the net charge of the. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Do you want to learn how to build an atom from scratch?
Swipe left and right to view this activity. Electron configuration can be done in two ways. Chlorine has a major role to play in synthetic organic chemistry, taking part in three of the most common reaction mechanisms.
Color the moved dot red. We’ll use a bohr diagram to visually represent where the electrons. On earth, chlorine forms molecules of two chlorine atoms.
One single bond between atoms and three lone pairs of. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) chlorine (cl) atom. You can also play a fun.
It is a pale green gas. The other halogen molecules (f 2, br 2, i 2, and at 2) form bonds like those in the chlorine molecule: Chlorine is a diatomic molecule and contains only two chlorine atoms.
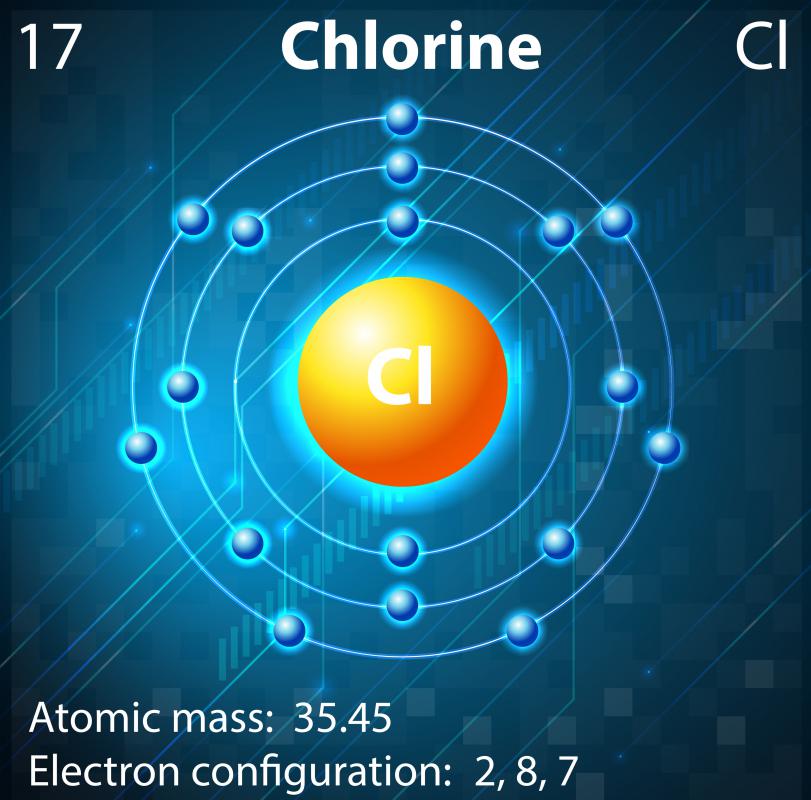
Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The atomic number of chlorine is 17.
This creates a sodium cation and a chlorine anion. Draw an arrow that indicates the dot has moved from the calcium to the chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −).

/chlorineatom-58b602515f9b5860464c5c02.jpg)